LGSOC Awareness Day: Breakthrough FDA Approval Marks Hope for 80,000 Women
LGSOC Awareness Day 2025: A breakthrough FDA approval proves rare cancers deserve focus, funding, and a future of hope.
This FDA approval proves what we’ve always believed: when rare cancers get the focus they deserve, breakthroughs follow.”
MIAMI, FL, UNITED STATES, September 9, 2025 /EINPresswire.com/ -- September 9 marks the second annual Low-Grade Serous Ovarian Cancer (LGSOC) Awareness Day. But 2025 isn't just another awareness campaign. It’s the year a community of advocates came together to make change.— Emily Campbell
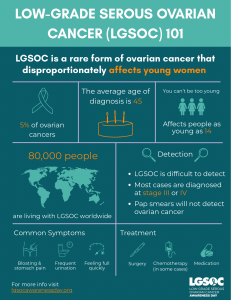
Low-grade serous ovarian cancer affects 6,000 to 8,000 women in the U.S. and 80,000 worldwide every year. LGSOC strikes younger women with a median age of 45 versus 63 for high-grade ovarian cancer, yet it has historically received a fraction of research attention and funding.
The research tells a stark story: chemotherapy for LGSOC has low success rates — only 4% of patients responded when treated before surgery and 25% when treated after surgery. For every 100 people diagnosed, more than 80 will have their cancer come back. Yet until this year, there were zero FDA-approved treatments specifically designed for LGSOC.
At Not These Ovaries, we see these numbers as more than statistics. They represent thousands of women who deserved better, faster answers.
The moment that changed everything
Last May, medical history was made. The FDA granted accelerated approval to avutometinib and defactinib (Avmapki Fakzynja Co-Pack), the first-ever treatment approved for LGSOC patients, specifically for recurrence with KRAS mutations.
But here's what's critical: this approval comes with specific requirements. The FDA approved this treatment specifically for adult patients who meet the following criteria:
- Have low-grade serous ovarian cancer (LGSOC)
- Have recurrent disease (cancer that has returned after prior treatment)
- Have a confirmed KRAS mutation in their tumor (this is a critical requirement)
- Have received prior systemic therapy
Without a confirmed KRAS mutation, patients cannot access this breakthrough treatment. This makes genetic testing not just helpful, but crucial for treatment decisions.
The Phase 2 RAMP 201 trial demonstrated a remarkable 44% overall response rate in KRAS-mutated patients, with responses lasting anywhere from 3.3 to 31.1 months. This breakthrough targets approximately 30% of LGSOC patients who carry KRAS mutations, transforming their journey from "managing with treatments designed for other cancers" to accessing purpose-built therapy.
Why this matters beyond the medicine
This isn't just about one drug. It's validation that LGSOC deserves recognition as a distinct disease requiring specialized research and targeted treatments.
For too long, LGSOC patients received what we call "treatment leftovers" and off-label use of medication: therapies designed for other cancers, with limited effectiveness. This approval proves that when you fund research specifically for rare diseases, breakthroughs follow. And when patient communities partner with researchers who listen, we can accelerate progress that seemed impossible just years ago.
What comes next
The ongoing Phase 3 RAMP 301 trial is evaluating this combination in broader LGSOC populations, both with and without KRAS mutations. This could potentially expand treatment options for even more patients, which is exactly why continued research funding matters.
For the 90% of LGSOC patients who report that their lives "revolve around cancer," this approval offers something revolutionary: the possibility that effective treatment might allow life to revolve around living instead.
Action steps for LGSOC Awareness Day
This LGSOC Awareness Day, here's how you can drive real change:
- Share life-saving awareness: The symptoms of LGSOC — persistent bloating, pelvic pain, feeling full quickly, urinary urgency, and abdominal discomfort — shouldn't be dismissed, especially in younger women. Share these symptoms widely.
- Fund research that happens now: Organizations like Not These Ovaries fund clinical trials that start immediately. Your donation today goes 100% toward research, not overhead.
- Advocate for genetic testing: KRAS mutation testing is now critical for LGSOC treatment decisions. Ensure patients know to request comprehensive tumor profiling, not just basic genetic screening.
- Connect patients with specialists: Link people to gynecologic oncologists experienced in rare ovarian cancer subtypes and LGSOC-specific support networks.
Action and understanding will save lives
Today, we celebrate a medical milestone that seemed impossible just years ago. Tomorrow, we continue working toward a future where no LGSOC patient faces their diagnosis feeling alone, uninformed, or without hope.
The approval of avutometinib and defactinib proves our core belief: when rare diseases receive focused attention and urgent funding, breakthrough treatments follow. This LGSOC Awareness Day, we're not just raising awareness; we're demonstrating that advocacy, research, and swift action can literally save lives.
The question isn't whether we can develop treatments for rare cancers. This approval proves we can. The question is: how fast can we fund the next breakthrough?
Chris Campbell
Not These Ovaries
impact@nottheseovaries.org
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
X
Frequently Asked Questions about LGSOC
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.