Gene Therapy for Rare Disease Market Size to Reach $24.54B By 2033 | DataM Intelligence
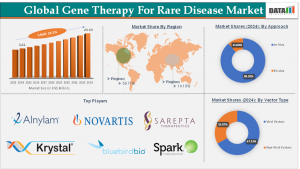
Gene Therapy for Rare Disease market is projected to grow from $3.01B in 2024 to $24.54B by 2033, with a CAGR of 26.5% driving innovative treatments
Driven by rising rare disease prevalence and advanced biotech, the US Gene Therapy market is set to grow rapidly, contributing significantly to the global $23.5Bn forecast by 2032.”
AUSTIN, TX, UNITED STATES, May 26, 2025 /EINPresswire.com/ -- Gene Therapy for Rare Diseases: Market Perspective and Key Advances in 2025— DataM Intelligence
Gene therapy has emerged as a transformative approach in treating rare diseases, offering potential cures by addressing the root genetic causes. As of 2025, the global market for gene therapies targeting rare conditions is experiencing significant growth, driven by technological advancements, increased investment, and a deeper understanding of genetic disorders.
Market Value and Growth
In 2025, the Gene Therapy For Rare Disease Market Size is valued at approximately US$ 3.01 Billion in 2024, marking a substantial increase from previous years. This growth trajectory is expected to continue, with projections indicating a market size of US$ 24.54 Billion by 2033. The compound annual growth rate (CAGR) stands at an impressive 26.5%, underscoring the rapid expansion of this sector.
Several factors contribute to this growth:
Technological Advancements: Innovations in gene-editing tools, such as CRISPR-Cas9, have streamlined the development of gene therapies, making them more efficient and targeted.
Regulatory Support: Regulatory bodies worldwide are providing frameworks that facilitate the approval and commercialization of gene therapies, recognizing their potential in addressing unmet medical needs.
Increased Investment: Both public and private sectors are investing heavily in gene therapy research and development, recognizing the long-term benefits and potential returns.
Regional Outlook
North America: Holding a significant share of the global market, North America continues to lead in gene therapy research, clinical trials, and commercialization. The presence of major biotech firms and supportive regulatory environments contribute to this dominance.
Europe: European countries are actively investing in gene therapy research, with several collaborative projects and funding initiatives aimed at accelerating development and access.
Asia-Pacific: This region is witnessing rapid growth, driven by increasing healthcare expenditures, rising awareness of rare diseases, and supportive government policies. Countries like Japan and China are at the forefront of this expansion.
Key Players in the Market
Spark Therapeutics, Inc.
Novartis AG
bluebird bio, Inc.
Ferring Pharmaceuticals Inc.
Vertex Pharmaceuticals Incorporated
Sarepta Therapeutics, Inc.
CSL Behring LLC
Alnylam Pharmaceuticals, Inc.
Amgen, Inc.
Orchard Therapeutics group.
Krystal Biotech, Inc.
Market segmentation:
By Vector Type: Viral Vectors, Non-Viral Vectors
By Technique: Gene Addition, Gene Silencing, Gene Editing
By Approach: In-Vivo, Ex vivo
By Application: Musculoskeletal Conditions, Blood Disorders, Oncology, Ophthalmology, Others
By Region: North America, Europe, South America, Asia Pacific, Middle East, and Africa
Latest Developments in the USA
The United States remains a hub for gene therapy advancements:
FDA Approvals: The U.S. Food and Drug Administration (FDA) has approved several gene therapies for rare diseases, including treatments for spinal muscular atrophy and certain types of inherited blindness.
Pricing Trends: A recent analysis revealed that prices for new drugs in the U.S. have more than doubled over the past four years, largely due to the focus on rare diseases. In 2024, the typical yearly list price for a newly launched medication exceeded $370,000, nearly doubling from $180,000 recorded in 2021. This trend coincides with an increase in orphan drug launches, which constituted 72% of new U.S. drug approvals in 2024.
Research Initiatives: Numerous clinical trials are underway, exploring gene therapies for conditions like sickle cell disease, hemophilia, and various metabolic disorders.
Latest Developments in Japan
Japan is making remarkable strides in the gene therapy arena:
Market Growth: The country's gene therapy market is forecasted to rise from $218.1 million in 2025 to approximately $872.4 million by 2035, registering a steady compound annual growth rate (CAGR) of about 13.4%. This growth is fueled by increasing collaborations among biotech companies, rising investments in research, and supportive government measures.
Regulatory Advancements: Japan's regulatory bodies are streamlining approval processes for gene therapies, encouraging faster access to innovative treatments.
Collaborative Efforts: Japanese institutions are partnering with global biotech firms to co-develop gene therapies, enhancing the country's position in the global market.
Conclusion
The gene therapy market for rare diseases is poised for remarkable growth in the coming years. With advancements in technology, supportive regulatory environments, and increasing investments, gene therapies are transitioning from experimental treatments to mainstream solutions for previously untreatable conditions. As the market evolves, stakeholders must navigate challenges related to pricing, accessibility, and ethical considerations to ensure that the benefits of these therapies reach all patients in need.
Latest Related Report By DataM Intelligence
US Gene Therapy Market Size 2025-2033
Cell and Gene Therapy Manufacturing Services Market Size 2025-2033
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.