Decoding bone health: single-cell insights into type 2 diabetes
GA, UNITED STATES, March 7, 2025 /EINPresswire.com/ -- A pioneering study offers unprecedented insights into the bone immune microenvironment of type 2 diabetic mice, uncovering a unique genetic profile that includes decreased osteoclast differentiation. This research is pivotal as it sheds light on the cellular and molecular mechanisms that could be targeted to address bone health complications associated with type 2 diabetes, potentially leading to novel therapeutic interventions.
When we think of type 2 diabetes, images of heart problems or kidney damage often come to mind. But lurking quietly behind these well-known complications is another serious threat—bone disease. Affecting over 90% of global diabetes cases, type 2 diabetes doesn't just compromise vital organs; it takes a hidden toll on the skeleton, making bones more prone to fractures and slow to heal. Despite this, the connection between diabetes and bone health has been a neglected field. With millions of patients at risk, scientists are racing against time to unearth the secrets of how diabetes weakens our bones.
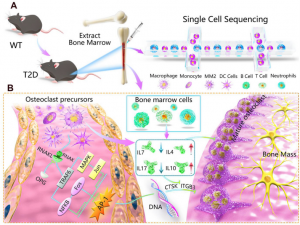
That's precisely what a research team from the Southern University of Science and Technology set out to do. Published (DOI: 10.1016/j.gendis.2023.101145) on October 17, 2023, in Genes & Diseases, their study brings fresh insights to light. By employing cutting-edge single-cell RNA sequencing on bone marrow from a mouse model of type 2 diabetes, the team created an intricate map of the bone immune microenvironment. What they discovered was nothing short of a revelation: diabetes disrupts the genetic orchestra inside our bone marrow, reshuffling immune cell behavior and slowing down the natural bone remodeling process.
The study identified 21 distinct cell clusters within the bone marrow, with monocytes, neutrophils, and B lymphocytes dominating the scene. But a particularly concerning discovery was a spike in a monocyte/macrophage subset marked by Cd36. These cells, crucial for healthy bone turnover, were found to have reduced potential for transforming into osteoclasts—the cells responsible for bone resorption. Without proper osteoclast activity, bones can't renew themselves effectively, setting the stage for fragility and fractures. Further analysis revealed that diabetes tampered with key cytokine networks and downregulated the AP-1 transcription factor, a vital driver of osteoclast formation.
"Our research provides a comprehensive cellular map of how diabetes disrupts bone immune interactions," explained Dr. Lin Wang, one of the study's authors. "By identifying critical molecular pathways, such as the suppression of AP-1 in osteoclast differentiation, we move closer to understanding the skeletal complications of diabetes. This work lays the groundwork for potential therapeutic strategies to counteract bone fragility in diabetic patients."
The implications of these findings are profound. As scientists continue to decode the intricate interplay between bone and immune cells, potential treatments could emerge to counter the bone-weakening effects of diabetes. By targeting the disrupted cytokine networks or boosting osteoclast activity, researchers hope to develop therapies that can prevent fractures and promote faster bone healing. For now, the study stands as a crucial step toward safeguarding the skeletal health of millions worldwide, highlighting a new frontier in the battle against diabetes.
DOI
10.1016/j.gendis.2023.101145
Original Source URL
https://doi.org/10.1016/j.gendis.2023.101145
Funding information
This work was supported by the National Natural Science Foundation of China (No. 81972045), the Basic Applied Basic Research Foundation of Guangdong Province, China (No. 2022A1515012373), and the research fund from SUSTech Hospital.
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.